Related Posts



You are about to leave Risk Strategies website and view the content of an external website.
You are leaving risk-strategies.com
By accessing this link, you will be leaving Risk Strategies website and entering a website hosted by another party. Please be advised that you will no longer be subject to, or under the protection of, the privacy and security policies of Risk Strategies website. We encourage you to read and evaluate the privacy and security policies of the site you are entering, which may be different than those of Risk Strategies.

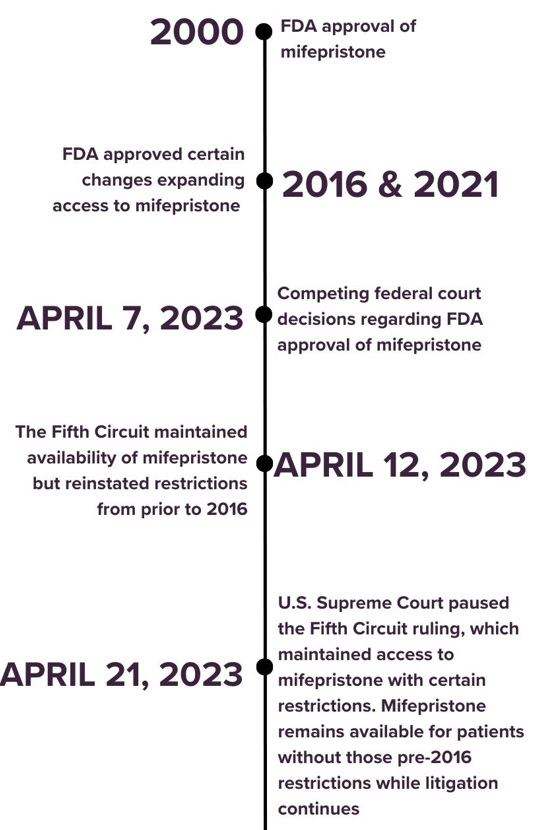
On Friday, April 21, 2023, the Supreme Court issued a decision to maintain widespread access to mifepristone, a medication whose primary intent is to aid in medical termination of early pregnancy.
This most recent decision by the Supreme Court blocked two lower court rulings that would have limited access to mifepristone, a medication first approved by the Federal Drug Administration (FDA) in 2000 under a pathway used for medications that treat serious or life-threatening illnesses.
In 2016 and 2021, the FDA approved certain changes to mifepristone including:
On April 7, 2023, two federal courts issued competing decisions regarding mifepristone access. A Texas district court suspended the FDA’s approval of mifepristone, restricting access on a nationwide basis. A Washington district court simultaneously affirmed the FDA’s approval of mifepristone and blocked the government from restricting access to the medication in 17 states and Washington, D.C.
On April 12, 2023, the US Court of Appeals for the Fifth Circuit ruled that the Texas court decision was partially overturned; this ruling maintained access to mifepristone but reinstated restrictions from prior to 2016.
The Supreme Court’s April 21, 2023 ruling allows the current FDA rules to remain in effect, maintaining mifepristone availability as litigation continues. The Fifth Circuit is scheduled to hear the merits of the preliminary injunction (the temporary block of mifepristone’s FDA approval) on May 17, 2023, which will be the next step as this case continues through the courts. In the meantime, access to mifepristone remains available.
See below for a general timeline of events around access to mifepristone from its FDA approval in 2000 to the present day.
Risk Strategies will continue to closely monitor developments in this space and will provide updates when available. Contact us directly at benefits@risk-strategies.com.
The contents of this article are for general informational purposes only and Risk Strategies Company makes no representation or warranty of any kind, express or implied, regarding the accuracy or completeness of any information contained herein. Any recommendations contained herein are intended to provide insight based on currently available information for consideration and should be vetted against applicable legal and business needs before application to a specific client.


